Every year, the FDA issues over 1,200 safety alerts about medications - some about contaminated batches, others about dangerous side effects or sudden shortages. If you're a patient, caregiver, or healthcare worker, missing one of these alerts could mean taking a risky drug without knowing it. The good news? You can get these alerts delivered straight to your inbox - for free. The bad news? Most people don’t know how, or even that they exist.
What the FDA Alerts Actually Cover
The FDA doesn’t just warn about recalled drugs. Their alerts cover three main types of safety issues: recalls, serious side effects, and label changes. For example, in 2023, an alert warned about a batch of insulin that had degraded due to improper storage. Another flagged a generic blood pressure medication contaminated with a cancer-causing chemical. These aren’t rare events. In fact, the FDA issued 1,427 drug safety notifications in 2023 alone.
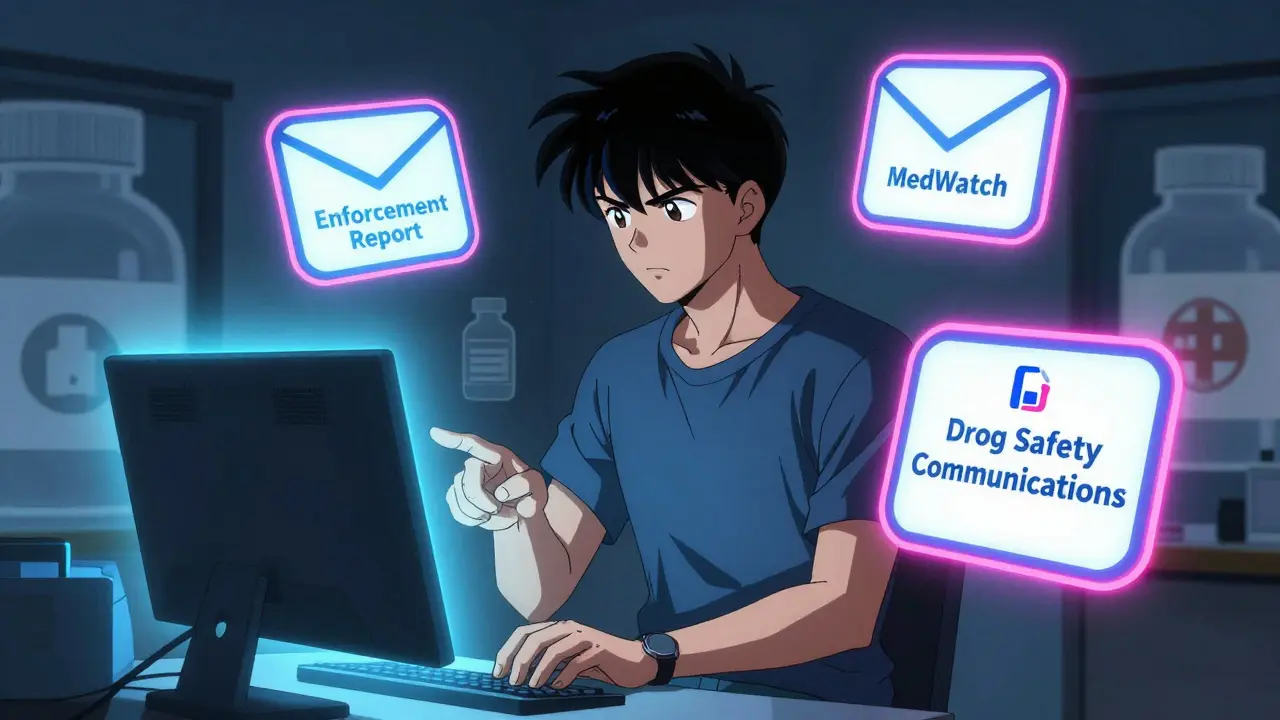
There are three separate systems, each serving a different purpose:
- Enforcement Report Subscription Service - Sends emails when a product is recalled. This includes prescription drugs, over-the-counter meds, and even medical devices.
- MedWatch Safety Alerts - Covers serious adverse events, labeling changes, and safety communications. This is where you’ll learn about newly discovered risks like heart problems or liver damage linked to a drug.
- Drug Safety Communications - Focuses on alerts about specific drug classes. Think: "all SSRI antidepressants may increase bleeding risk" or "new warnings for diabetes drugs in elderly patients."
These aren’t the same thing. A recall doesn’t always mean a drug is unsafe - sometimes it’s just a labeling error or a manufacturing flaw. But a Drug Safety Communication means the FDA has found real, documented harm. You need both.
How to Subscribe - Step by Step
Signing up takes less than five minutes per system. Here’s how to set each one up.
1. Enforcement Report Subscriptions (Recalls)
Go to fda.gov/enforcement-report-subscription. Enter your email address. Then, check the boxes for the product types you care about. If you’re a patient, select Drugs. If you manage medications at a clinic or pharmacy, check Medical Devices too.
Next, pick your frequency: daily or weekly. Daily is best if you’re a pharmacist or hospital staff. Weekly works for most patients.
Here’s the game-changer: you can add up to five custom keywords. Type in "insulin," "metformin," "warfarin," or even "peanut" if you have allergies. You’ll only get alerts with those words. No more sifting through recalls for baby formula or toothpaste.
2. MedWatch E-list (Safety Alerts & Adverse Events)
Visit fda.gov/medwatch-email-list. Fill out the form with your name and email. That’s it. You’ll start getting emails from MedWatch - usually 2-4 times a week.
These alerts are more clinical. They might say: "New warning added to label for drug X: risk of pancreatitis in patients with history of gallstones." This is the info your doctor needs to know before prescribing.
You can also follow @FDAMedWatch on Twitter for real-time updates. Over 285,000 people do. It’s not a replacement for email - but it’s useful for breaking news.
3. Drug Safety Communications (Drug Class Warnings)
Go to fda.gov/drugs/drug-safety-and-availability/drug-safety-communications. Scroll down and click Sign up for email alerts. You’ll get a confirmation email. Click the link. Done.
This system is especially helpful if you take a drug from a class with known risks - like statins, NSAIDs, or antipsychotics. You’ll get a single email when the FDA updates safety info for your entire drug category, not just one brand.
Why Most People Miss These Alerts
A 2022 government report found only 38% of doctors knew all three systems existed. That’s shocking. And it’s even worse for patients. A 2023 survey showed only 17% of consumers were subscribed to any FDA alert.
Why? Three reasons:
- Confusion - People think one system covers everything. It doesn’t. Recalls ≠ safety warnings.
- Overload - Some get so many emails they turn them off. The FDA’s own survey found 28% of subscribers want better filtering.
- Assumption - Many believe their pharmacist or doctor will tell them. But pharmacies don’t always get alerts before patients do.
One hospital pharmacist in Ohio told a Reddit thread: "I got the insulin recall alert on a Friday. We had two vials left. We pulled them before anyone got them. That alert saved us. I didn’t even know I was subscribed - until I saw the email."

What the FDA Doesn’t Do - And What You Need to Know
The FDA system is powerful, but it’s not perfect.
It doesn’t send push notifications. No app. No text alerts. If you’re not checking your email, you won’t see the warning.
It doesn’t explain risk in simple terms. An alert might say: "Increased risk of hepatotoxicity." That’s not helpful if you’re not a doctor. You’ll need to read the full document or call your provider.
And it doesn’t prioritize. A low-risk label change and a life-threatening recall both show up the same way. That’s why alert fatigue is real. One doctor said: "I get 12 emails a week. Half are "this pill’s expiration date was misprinted." I started ignoring them all."
That’s why keyword filtering is so important. If you only care about your blood thinner, set your keyword to "warfarin" or "apixaban." You’ll cut 80% of the noise.
Who Should Subscribe - And Who Shouldn’t
Here’s who benefits most:
- Patients on long-term meds - If you take something daily for years - diabetes, high blood pressure, depression - you need these alerts.
- Parents of kids with chronic conditions - Kids on ADHD meds, epilepsy drugs, or asthma inhalers are at higher risk for unexpected side effects.
- Pharmacists and nurses - You’re the last line of defense. These alerts help you catch errors before they reach the patient.
- Caregivers for elderly patients - Older adults often take 5-10 drugs. One new interaction can be deadly.
Who might not need it? If you only take occasional painkillers or vitamins, you probably don’t need to subscribe. But if you’re on a drug for more than three months, you’re a candidate.
What’s Coming in 2025
The FDA is fixing the biggest complaints. By mid-2025, they plan to:
- Launch a mobile app with push notifications
- Combine all three alert systems into one dashboard
- Double the number of keywords you can set - from five to ten
- Release Spanish-language alerts (finally)
They’re also testing AI to sort alerts by urgency. Right now, every alert is treated equally. Soon, the most dangerous ones will rise to the top.
Final Tip: Don’t Wait for a Crisis
People only sign up after something goes wrong. A friend gets sick. A news story breaks. Then they scramble to find the FDA site.
Don’t wait. Set up your alerts today. It takes less time than making a coffee. And if you’re already on multiple medications? It’s one of the safest, simplest things you can do for your health.
Start with the Enforcement Report. Add your drug names as keywords. Then sign up for MedWatch. Finally, get Drug Safety Communications. In three minutes, you’ve given yourself a shield against hidden drug risks.
And if you’re still unsure? Call the FDA’s Help Desk. They answer within two business days. No charge. No hassle. Just real help.
Are FDA drug safety alerts free?
Yes. All FDA drug safety alert systems - including Enforcement Reports, MedWatch, and Drug Safety Communications - are completely free. You only need an email address to subscribe. There are no hidden fees, no premium tiers, and no trials.
How often do I get alerts?
It depends on the system and your settings. Enforcement Reports can be sent daily or weekly - most people choose weekly. MedWatch sends 2-4 emails per week. Drug Safety Communications come less frequently, usually once a week or less, depending on new safety findings. You’ll get more alerts if you use broad categories or no keywords.
Can I get alerts in Spanish?
Not yet, but it’s coming. The FDA announced in 2024 that Spanish-language versions of all three alert systems will launch in Q3 2025. Until then, you can use browser translation tools on the FDA website to read alerts in Spanish.
What’s the difference between a recall and a safety alert?
A recall means a product is removed from the market because it’s defective, contaminated, or mislabeled - not necessarily dangerous. A safety alert means the FDA has found new evidence that a drug can cause harm, even if it’s made correctly. You can have a safety alert without a recall - and vice versa. Both matter.
Can I unsubscribe later?
Yes. Every alert email has an unsubscribe link at the bottom. You can turn off any system at any time. You can also update your keywords or change your frequency without unsubscribing entirely.
Do these alerts apply to over-the-counter drugs?
Yes. The FDA’s Enforcement Report system covers all FDA-regulated products, including OTC pain relievers, allergy meds, antacids, and even herbal supplements. If it’s sold in the U.S. and regulated by the FDA, it’s included.
Is the FDA the only source for drug safety info?
No, but it’s the most authoritative. Commercial services like MedWatcher or First Databank offer alerts too, but they usually only cover prescription drugs and charge fees. The FDA covers everything - including generics, biologics, and medical devices - and it’s the only system backed by federal law. Always cross-check with FDA alerts first.
What if I miss an alert? Can I look up past ones?
Yes. All past Enforcement Reports, MedWatch alerts, and Drug Safety Communications are archived on the FDA website. You can search by drug name, date, or keyword. The archives go back to 2010. If you’re worried about a medication you took last year, check the archive - you might find a warning you never saw.


Ed Mackey
February 3, 2026 at 00:48Just signed up for all three alerts. Took me 4 minutes. I take metformin and warfarin so I added those keywords. Finally feel like I’m not flying blind with my meds. Thanks for this.
Kunal Kaushik
February 4, 2026 at 06:29Bro this is 🔥. I’m in India and didn’t even know the FDA did this. Just subscribed. My mom’s on blood pressure meds - she’s gonna be so glad I found this. 🙏
Mandy Vodak-Marotta
February 5, 2026 at 17:10Okay but can we talk about how wild it is that 83% of patients don’t know these exist? I’m a nurse and I used to think my pharmacy would tell me if something was sketchy - spoiler: they don’t. I got an alert about a contaminated batch of lisinopril last year and I literally screamed because my grandma was on it. Now I have all three alerts on my phone and I check them like a morning coffee. Also, the keyword trick? Genius. I only get alerts for ‘duloxetine’ and ‘oxycodone’ now. My inbox went from spam to useful. If you’re on more than one med, do this. Seriously.
Nathan King
February 7, 2026 at 14:52The structural integrity of the FDA’s pharmacovigilance framework is commendable, though its dissemination remains critically underutilized. The tripartite alert architecture - Enforcement Reports, MedWatch, and Drug Safety Communications - constitutes a robust, multi-layered surveillance mechanism that ought to be institutionalized in clinical training curricula. The fact that only 17% of consumers subscribe reflects a systemic failure in public health literacy, not a deficiency in the system itself.
Harriot Rockey
February 8, 2026 at 18:42This is so important!! 🌟 I’m a caregiver for my dad who’s on 7 meds and I had NO IDEA about any of this. I just subscribed to all three and set keywords for his drugs - I’m crying a little because I feel like I just gave him a superpower. Thank you for writing this like you actually care. Also, the part about the pharmacist in Ohio? That’s the kind of story that keeps me going. 💙
Samuel Bradway
February 10, 2026 at 05:29My pharmacist didn’t even know about the keyword thing. I told her and she said ‘wow, I’m stealing this.’ Just added ‘metoprolol’ and ‘levothyroxine’ to mine. Life changed.
pradnya paramita
February 10, 2026 at 18:54It’s worth noting that MedWatch also accepts spontaneous adverse event reports from the public - if you experience something unusual, file it. That’s how the FDA detects signals early. The FDA’s FAERS database is public, and your report could help flag a class-wide risk. Don’t just subscribe - participate. Your anecdote might be the data point that saves someone else.
Rachel Kipps
February 12, 2026 at 11:44I signed up… then forgot. Got an alert last week about a recall I missed. I’ve been taking that drug for 5 years. I’m signing up again. And I’ll actually read them this time.
Prajwal Manjunath Shanthappa
February 12, 2026 at 16:20Ugh. Another ‘how-to’ post that assumes everyone has access to email, a computer, and the cognitive bandwidth to navigate federal bureaucracy. Meanwhile, my aunt - who takes 8 meds, doesn’t speak English, and lives on Social Security - is still getting pills from a 7-Eleven pharmacy with no alerts, no translator, no nothing. This ‘solution’ is for privileged people who have time to ‘subscribe.’
Wendy Lamb
February 14, 2026 at 09:30Done. Three minutes. No drama. Just did it. You should too.
Antwonette Robinson
February 14, 2026 at 21:53Wow. So you’re telling me I should actually pay attention to my meds instead of just trusting the system? Who knew? 😏
Katherine Urbahn
February 15, 2026 at 12:05It is morally indefensible that the FDA does not proactively notify patients via SMS or mobile app - and yet, here we are, relying on email, which is, frankly, archaic. Furthermore, the lack of standardized risk categorization renders these alerts nearly useless to the layperson. This is not empowerment - it is burden-shifting onto the patient.
Alex LaVey
February 15, 2026 at 19:38I’m from the U.S. but my mom’s from Mexico - she’s on a statin and doesn’t read English. I printed out the FDA alerts in Spanish and taped them to her fridge. We’re waiting for the 2025 Spanish version, but until then? Google Translate + paper works. This stuff matters. Don’t let language be a barrier.
caroline hernandez
February 16, 2026 at 23:45As a clinical pharmacist, I’ve seen firsthand how Drug Safety Communications prevent polypharmacy disasters. One alert on SSRI bleeding risk saved a 78-year-old from a GI bleed - she was on aspirin, warfarin, and sertraline. The FDA’s system isn’t perfect, but it’s the only federally mandated, evidence-based pharmacovigilance tool we have. Use it. Share it. Advocate for it.
Jhoantan Moreira
February 17, 2026 at 04:37Just subscribed. I’m in the UK, but I’ve got family in the States on chronic meds. Forwarded this to my cousin. She’s 72 and on 6 pills. She didn’t even know this existed. Thanks for making it simple. 🙏❤️