Ever wonder why some drugs have long, weird names like omeprazole or adalimumab, while others are called something simple like Prilosec or Humira? It’s not random. Every drug has three names - and each one serves a different purpose. Understanding them isn’t just for pharmacists or doctors. It’s for anyone who takes medication - because getting the name wrong can lead to dangerous mistakes.
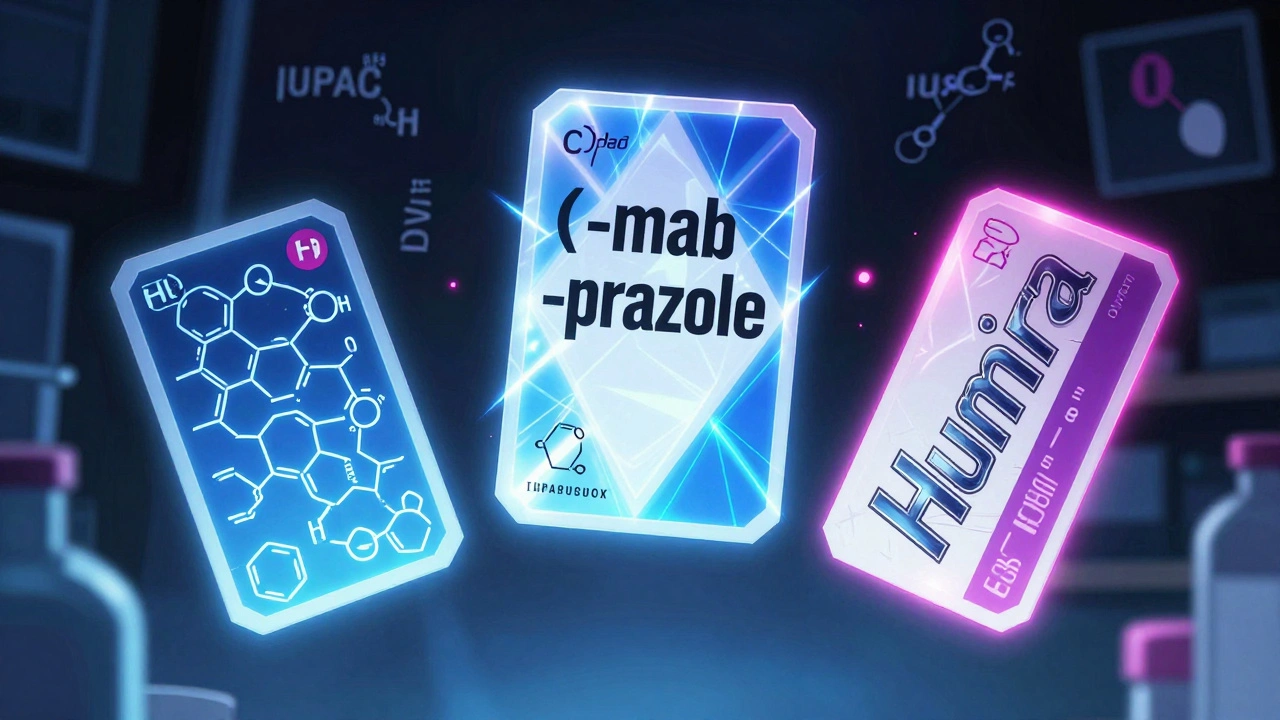
Chemical Names: The Science Behind the Formula
The most precise name for any drug is its chemical name. This is built using rules from the International Union of Pure and Applied Chemistry (IUPAC). It tells you exactly how the atoms are arranged - like a molecular blueprint. For example, the chemical name for the heart drug propranolol is 1-(isopropylamino)-3-(1-naphthyloxy)propan-2-ol. That’s 52 characters long. Try saying that at the pharmacy.
Chemical names are useful in labs, patents, and research papers. But no doctor writes them on a prescription. No pharmacist stocks shelves using them. They’re too long, too complex, and too hard to remember. Imagine if every time you needed insulin, you had to ask for hexadecanoic acid, 2-(4-hydroxyphenyl)ethyl ester. It would slow down everything - and increase errors.
Still, chemical names matter. They’re the foundation. Every generic and brand name starts here. If you’re reading a scientific study or checking a drug’s structure, this is the name you need. But for daily use? You’ll never use it.
Generic Names: The Universal Language of Medicine
This is where things get practical. Generic names - also called nonproprietary names - are the standardized labels used worldwide. They’re assigned by official bodies: the World Health Organization (WHO) through its International Nonproprietary Names (INN) program, and in the U.S., the United States Adopted Names (USAN) Council.
Here’s the key: generic names aren’t random. They follow patterns. The ending - called a stem - tells you the drug’s class and how it works.
- Drugs ending in -prazole (omeprazole, pantoprazole) are proton pump inhibitors - used for acid reflux.
- Those ending in -tinib (imatinib, sunitinib) are tyrosine kinase inhibitors - used in cancer treatment.
- Drugs ending in -mab (adalimumab, rituximab) are monoclonal antibodies - often for autoimmune diseases.
- Newer ones like -siran (for RNA therapies) and -dutide (for peptide-drug conjugates) are being added as science advances.
The prefix - the part before the stem - is chosen to make each drug unique. It’s not random either. The USAN Council rejects about 30% of proposed generic names because they sound too similar to existing ones. Why? To prevent mix-ups. A single letter or sound can cause a fatal error. One study found that properly designed generic names reduce medication errors by 27%.
These names are used on prescriptions, in medical records, and on drug labels. They’re the same no matter which country you’re in. That’s why a doctor in Tokyo and one in Toronto can both write metformin and know they’re talking about the same drug. That’s global safety in action.
Brand Names: The Marketing Side of Medicine
Brand names - also called trade or proprietary names - are what pharmaceutical companies create to sell their drugs. Think Prilosec, Humira, Viagra. These are catchy, memorable, and protected by trademarks.
But getting a brand name approved isn’t easy. Companies submit 150 to 200 options to the FDA. The FDA’s Medication Errors team reviews each one. About one in three gets rejected. Why? Because of confusion risk.
The FDA has strict rules: brand names can’t:
- Sound like another drug (e.g., Hyzaar vs. Hytrin)
- Look too similar in writing (e.g., Epivir vs. Epivir-HBV)
- Make medical claims (no names like QuickCure or HeartFix)
- Use the same stem as the generic name unless it’s clearly different
Even the color, shape, and size of the pill matter. A brand-name drug can’t look identical to its generic version - that’s a trademark rule. But here’s the catch: the active ingredient is exactly the same. That’s why generics cost less. They don’t pay for marketing, ads, or fancy packaging.
Still, patients get confused. A 2022 FDA survey found that 68% of patients don’t understand why their pill looks different when they refill a prescription. They think it’s a different drug. Pharmacists see this every day. But 83% of them say standardized naming helps avoid errors - even if patients don’t realize it.

Company Codes: The Secret Names During Development
Before a drug even reaches the clinic, it has an internal code. Pfizer uses PF-04965842-01. Merck uses MK-8668. These codes are made up of letters and numbers. The prefix often stands for the company. The digits track the compound, salt form, and batch.
These names are never used in public. They’re for lab notebooks, clinical trial documents, and regulatory filings. But they’re critical behind the scenes. They help scientists track the drug through years of testing - from petri dishes to human trials.
When a drug finally gets a generic name, the company code is retired. But if the drug fails in trials, that code might sit in a database forever - a ghost of a drug that never made it to market.
Why This System Exists: Safety First
This whole system - chemical, generic, brand - exists because of one thing: preventing mistakes.
The Institute of Medicine found that at least 1.5 million preventable drug errors happen in the U.S. every year. Some are because of handwriting. Others are because names sound alike. Hydralazine vs. Hydroxyzine. One treats high blood pressure. The other treats allergies. Mix them up, and someone could get seriously hurt.
Since 2010, the WHO says standardized naming has reduced international medication errors by 18.5%. That’s not just a number. That’s lives saved.
Now, the system is evolving. With new kinds of drugs - like RNA therapies and targeted protein degraders - new stems are being created. AI tools now scan 15,000 existing drug names in seconds to check for similarities. The USAN Council says this cut confusion risks by 42% in its first year.
And it’s not slowing down. Experts predict that by 2030, over 4% of new drugs will be protein degraders - and they’ll all end in -tecan. That’s the future of naming.

What You Should Know as a Patient
Here’s what matters to you:
- Your prescription will list the generic name - that’s the active ingredient. Always check it.
- Your pill might look different if you switch from brand to generic. That’s normal. The active ingredient is the same.
- If you’re confused, ask your pharmacist. Say: “Is this the same as the one I took before?” They’ll check the generic name.
- Don’t assume that because a drug has a fancy brand name, it’s better. Generics are just as effective - and cost 80% less on average.
Most people don’t know this, but you have the right to ask for the generic version - unless your doctor says otherwise. It’s legal. It’s safe. And it saves money.
The Bigger Picture
Drug naming isn’t just about labels. It’s a global safety net. Every time a new drug is approved, scientists, regulators, and pharmacists work together to make sure its name doesn’t cause harm. That’s why the same name is used in 150 countries. That’s why a nurse in Nairobi can give a patient the right dose without knowing English.
It’s a quiet system. You don’t notice it - until something goes wrong. And that’s exactly how it should be. Like the brakes on your car, you only think about it when it works.
Next time you pick up a prescription, look at the name. Is it the generic? Is it the brand? Do they match what your doctor wrote? That small check could prevent a mistake - and maybe even save your life.


Rudy Van den Boogaert
December 5, 2025 at 04:39Really appreciated this breakdown. I never realized how much thought goes into naming drugs - it’s not just marketing, it’s literally a safety protocol. The stem system is genius. I’ve had my grandma mix up her meds before, and this explains why clear naming saves lives.
Jordan Wall
December 7, 2025 at 03:08Oh, for chrissakes, the IUPAC nomenclature is a masterpiece of linguistic austerity - a true testament to the sublime rigor of chemical formalism. Meanwhile, ‘Prilosec’? A marketing ploy disguised as pharmacology. 🤦♂️ The WHO’s INN system is the only thing keeping this entire edifice from collapsing into a sea of ‘CureAll-9000’ nonsense. And yes, I’ve read the FDA’s 2017 naming guidelines. Twice.
Pavan Kankala
December 8, 2025 at 12:03They’re lying. All of it. The ‘generic name’ is just a cover. Big Pharma controls the stems. They’re slowly replacing real medicine with coded branding - that’s why new drugs all end in ‘-mab’ or ‘-siran’. It’s not science, it’s a global mind-control experiment. And don’t get me started on how the FDA approves names. They’re in cahoots with the pharmaceutical lobby. You think they let you name a drug ‘CancerKiller’? Nah - they want you to keep buying the $10k pill.
Martyn Stuart
December 8, 2025 at 16:04Excellent post - really well-structured. I’d just add that the USAN Council’s rejection rate for similar-sounding names is even higher than stated - it’s closer to 35% now, thanks to AI screening. Also, don’t forget that some generic names are intentionally altered to avoid trademark conflicts in other languages. For example, ‘Citalopram’ became ‘Citalopram’ in the U.S., but ‘Citalopramum’ was rejected in Germany because it sounded like a type of sausage. Seriously.
Jessica Baydowicz
December 9, 2025 at 06:09OMG this is so cool!! I just learned that my blood pressure med is ‘amlodipine’ - and now I know it ends in ‘-dipine’ so it’s a calcium channel blocker 😍 I’m telling my whole family tonight. Also, I just asked my pharmacist for the generic version and saved $80!! Thank you for making science feel fun!! 💪💊
Shofner Lehto
December 9, 2025 at 17:56One thing people miss is that brand names are designed to be pronounceable across languages. Viagra? Easy in English, Spanish, Mandarin, Arabic. That’s not accidental. Companies hire linguists to test names in 20+ markets before submission. And yes, the FDA’s rejection rate for brand names is brutal - one company submitted ‘Vigora’ for erectile dysfunction and got rejected because it sounded too close to ‘Viagra’ in Spanish. They had to rename it ‘Zydena’.
Rachel Bonaparte
December 11, 2025 at 10:24Let me tell you something they don’t want you to know. The ‘generic’ name isn’t actually generic - it’s owned by the WHO, but the patent for the naming system? That’s held by a private consortium. The stems? They’re trademarked. That’s why new drugs can’t just pick their own endings. You think this is about safety? No - it’s about control. Who owns the language of medicine? Not you. Not me. The same people who own your insulin. And now they’re coding RNA therapies with ‘-siRNA’ stems - but only if you pay the licensing fee. That’s not science. That’s feudalism with a lab coat.
And don’t even get me started on how the FDA allows ‘Humira’ to be branded while the generic is ‘adalimumab’ - it’s psychological manipulation. They want you to believe the fancy name is better. It’s not. It’s just more expensive. And they know it.
I’ve been on this system for 12 years. I’ve seen the documents. They’re hiding the truth. The ‘stem’ system was designed to confuse patients into trusting the brand. And now AI is making it worse - algorithms are learning to make names sound ‘trustworthy’ - not accurate. That’s not innovation. That’s manipulation.
Next time you pick up a prescription, ask yourself: who benefits if you think this is complicated? Not you. Not your doctor. The people who profit from your confusion.
And yes, I’ve filed FOIA requests. I’m still waiting for the redacted pages.
Michael Feldstein
December 11, 2025 at 10:42That’s wild - I had no idea the -mab ending meant monoclonal antibody. Now I understand why my aunt’s rheumatoid arthritis med is called ‘rituximab’ and not something like ‘ArthritisStop’. Makes sense. I’m gonna start paying attention to the endings now - maybe I can even guess what a drug does before looking it up. Neat system.
Libby Rees
December 12, 2025 at 05:22This is one of the clearest explanations of drug naming I’ve ever read. Thank you. I’ve worked in international health for a decade, and I’ve seen patients in rural Kenya and rural Ohio confused by the same issue: the pill looks different, so they think it’s not the same. This post should be mandatory reading for every pharmacy tech and medical student.
Gillian Watson
December 12, 2025 at 12:38I just learned that my asthma inhaler is salbutamol. Never knew the -ol ending meant beta agonist. Cool.
Gareth Storer
December 12, 2025 at 22:36So let me get this straight - you’re telling me the reason my $200 brand-name drug looks different from the $20 generic is because the FDA doesn’t want them to look identical? That’s not safety. That’s corporate theater. Meanwhile, the active ingredient? Identical. The pill? Same chemistry. But I gotta pay extra because the logo’s different? Brilliant. 🙃