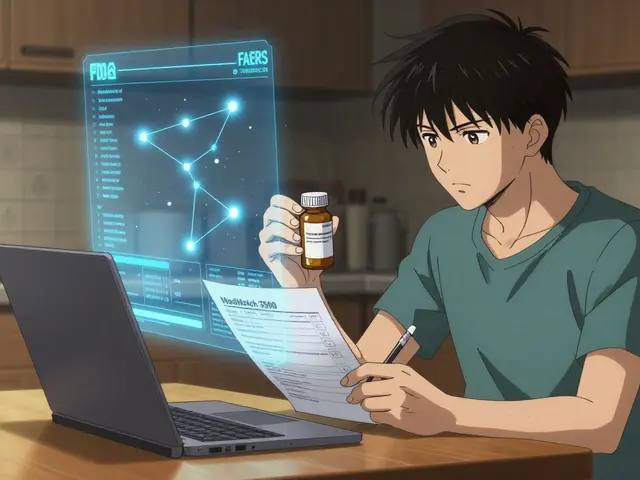
Every year, millions of people take prescription drugs, over-the-counter medicines, and even supplements without issue. But for some, a medication can cause an unexpected, harmful reaction. These are called adverse drug events. If you’ve experienced one-or witnessed one in a patient-you have the power to help protect others by reporting it to the FDA through MedWatch. This isn’t just paperwork. It’s how the FDA finds hidden dangers, updates warning labels, and sometimes pulls dangerous drugs off the market.
What Is MedWatch, Really?
MedWatch is the FDA’s official system for collecting reports about bad reactions to drugs, medical devices, vaccines, and other regulated products. It’s not a hotline for emergency help. It’s a safety net for the public. Every report adds to a massive database that scientists use to spot patterns. One report might seem small. But 100 similar reports? That’s a red flag. The system started in 1993 and now gets about 1.3 million reports a year. Most come from doctors and pharmacists. But anyone can report-patients, family members, caregivers. You don’t need to be sure the drug caused the problem. If something strange happened after taking a medication, report it. The FDA will sort out the rest.Who Should Report and What Needs Reporting?
You should report if you’re a:- Healthcare professional (doctor, nurse, pharmacist, dentist)
- Patient or family member
- Consumer who used a medication or supplement
- Severe rash after taking a new antibiotic
- Unexplained dizziness or fainting after starting a blood pressure pill
- Liver damage linked to a dietary supplement
- Swelling in the legs after using a new diabetes drug
- Psychiatric symptoms following use of an OTC sleep aid
Two Forms. Two Paths.
The FDA gives you two simple forms, depending on who you are.Form 3500: For Healthcare Professionals
This is a five-page form with 45 fields. It’s detailed because professionals have access to medical records. You’ll need:- Patient initials or medical record number (no full Social Security number)
- Name of the suspected drug(s)
- Exact dose and how it was taken (e.g., 20 mg orally daily)
- Date the drug was started and when the reaction happened
- Clear description of the event (what happened, how long it lasted)
- Outcome (did the patient recover? Was there hospitalization?)
- Any lab results, imaging, or other clinical data
- Whether stopping or restarting the drug changed the reaction
Form 3500B: For Patients and the Public
This is the simplified version-just three pages and 30 fields. It’s designed for people without medical training. You’ll still need:- Your name and contact info (optional but helpful)
- Patient’s age, sex, weight
- Drug name (brand or generic)
- How much and how often it was taken
- When you first noticed the problem
- What happened (use plain language: “My hands shook nonstop,” “I couldn’t stop vomiting”)
- What you did (did you stop the drug? Go to the ER?)
- Outcome: Did you get better? Still sick? Hospitalized?

How to Submit Your Report
There are four ways to send your report. Choose the one that’s easiest for you.1. Online (Fastest and Recommended)
Go to FDA.gov/MedWatch. Click “Voluntary Reporting.” You’ll be guided through an interactive form. You can save your progress and come back later. This is the most popular method-over 95% of reports now come in electronically.2. Fax or Mail
Download Form 3500 or 3500B from the FDA website. Fill it out, sign it, and mail or fax it to: FDA MedWatch5600 Fishers Lane
Rockville, MD 20857
Fax: 1-800-FDA-0178
3. Phone
Call 1-800-FDA-1088. A representative will take your report over the phone. The call center answers 95% of calls within 30 seconds. This is helpful if you’re not comfortable typing or if the reaction happened recently and you’re still processing what occurred.4. Mobile App (Pilot Program)
The FDA is testing a new app called MedWatch Express in 15 major teaching hospitals. It lets doctors submit reports directly from their phones using voice-to-text and pre-filled templates. If you’re a healthcare worker in one of these hospitals, ask your IT department about it. A public version is expected in 2026.What Happens After You Submit?
Once your report is in, the FDA doesn’t just file it away. Here’s what you can expect:- You’ll get an automated email confirmation within 24 hours.
- Within 21 days, you’ll receive a follow-up email with a unique case number.
- Your report joins a database of over 2 million annual reports.
- FDA scientists analyze patterns. If 10+ similar reports come in for the same drug, they investigate further.
- If a safety issue is confirmed, the FDA may update the drug label, issue a public warning, or even require a recall.
Common Mistakes and How to Avoid Them
Most people who report do it right. But here are the top errors-and how to skip them:- Waiting too long. Report within 15 days of noticing the event. The sooner, the better.
- Being too vague. Don’t just write “side effect.” Say “severe headache starting 2 hours after taking the pill, lasted 8 hours, relieved by Tylenol.”
- Leaving out drug details. If you took two new drugs at once, list both. The FDA needs to know what changed.
- Not reporting minor issues. Even if it wasn’t hospital-worthy, if it disrupted your life-like insomnia from an antidepressant-report it. These are the signals that predict bigger problems.
- Thinking it’s not your job. If you’re a patient, you’re the expert on your own body. Your report matters.

What MedWatch Doesn’t Cover
Not every bad reaction goes here:- Vaccines → Report to VAERS (vaccineadverseeventreporting.gov)
- Animal drugs → Report to the Center for Veterinary Medicine
- Food or cosmetic reactions → Report to the FDA’s Center for Food Safety and Applied Nutrition
- Medical devices → Yes, MedWatch covers them, but use the device-specific fields on Form 3500
Why Your Report Matters
The FDA estimates that only 1% to 10% of all adverse events are ever reported. That means for every 100 bad reactions, 90 to 99 go unnoticed. That’s dangerous. Doctors say they don’t report because it’s too time-consuming. But with EHR integration and the new mobile app, that’s changing. Patients say they don’t know how. That’s why you’re reading this now. Every report helps. A single report from a patient led to the removal of a popular weight-loss supplement linked to liver failure. Another helped identify a dangerous interaction between a common painkiller and a blood thinner. You’re not just reporting a bad reaction. You’re helping prevent someone else’s tragedy.Need Help? Resources You Can Use
- MedWatch Toll-Free: 1-800-FDA-1088 (Mon-Fri, 8 a.m.-5 p.m. ET)
- Online Training: MedWatch Learn (free 12-module course for professionals)
- Form Downloads: FDA.gov/MedWatch → “Forms” tab
- Mobile App Pilot: Available at 15 major U.S. teaching hospitals (expanding in 2026)
- QR Code Cards: Available at 30 major pharmacy chains-scan to report in 60 seconds




John Mackaill
November 22, 2025 at 07:20I reported my wife’s severe rash after her new statin last year. Didn’t think it mattered-turns out, three others had the same reaction. FDA added a warning two months later. You don’t need to be a doctor to save someone’s life. Just press submit.
It’s not paperwork. It’s solidarity.
Adrian Rios
November 23, 2025 at 04:33Let me tell you something that keeps me up at night: the FDA gets over a million reports a year, but that’s still less than 10% of what’s actually happening. Think about that. For every single report that makes it in, there are ten more people suffering in silence, convinced it’s ‘just how they are now’ or ‘old age’ or ‘bad luck.’
I work in a clinic. I’ve seen patients stop taking life-saving meds because they got a weird tingling and assumed it was ‘normal.’ They don’t report because they think it’s not ‘serious enough.’ But here’s the truth: the system doesn’t care if it’s serious-it cares if it’s *repeated*. One person? Maybe coincidence. Ten people with the same weird symptom? That’s a pattern. And patterns kill. And patterns get fixed. So if your knee started swelling after a new OTC painkiller? Report it. If your mom started hallucinating after a sleep aid? Report it. If your nephew got a rash after a supplement bought on Amazon? REPORT IT. Don’t wait for someone else to speak up. You’re not just reporting-you’re preventing the next headline. And honestly? If you’re reading this and you’ve never reported anything? You owe it to the next person who takes a pill.
Casper van Hoof
November 24, 2025 at 23:16The epistemological framework underlying MedWatch is predicated upon the aggregation of anecdotal evidence into a probabilistic model of pharmacovigilance. While the system’s efficacy is empirically validated through post-marketing surveillance metrics, its reliance on layperson reporting introduces a significant confounding variable: cognitive bias and linguistic imprecision. The translation of colloquial descriptors into standardized medical terminology, while commendable, remains an imperfect hermeneutic process. One must therefore question whether the democratization of adverse event reporting enhances signal detection or merely inflates noise.
Pramod Kumar
November 25, 2025 at 14:26Bro, I saw my cousin go from ‘just a little dizzy’ to ICU after one new pill. No one told her to report. She thought it was ‘just stress.’ We didn’t know any better. Now I tell everyone I know: if your body says ‘no,’ you don’t need a degree to say ‘report it.’
My aunt took a herbal tea for arthritis-ended up in the hospital with liver failure. We didn’t even know it was a drug. Turns out, ‘natural’ doesn’t mean ‘safe.’
That’s why I’m telling you this. Your grandma’s turmeric capsules? Your uncle’s muscle gainer? Your sister’s new antidepressant? If it changed how you felt-badly-tell the FDA. Don’t wait for a news story. Be the one who stops the next one.
It’s not hard. Three pages. Your words. Your power. Your people’s safety.
Just hit submit. 💪
Lisa Lee
November 27, 2025 at 11:48Why the hell are we letting some bureaucrat in Maryland decide what’s safe? We should just ban all drugs and let people live or die on their own. This is just more government overreach disguised as ‘safety.’
Jennifer Skolney
November 27, 2025 at 14:52Just reported my weird anxiety after that new OTC allergy med 😬
It was scary. Felt like my heart was trying to escape. Took me 5 minutes. Felt good to do it.
Thank you for making this so easy 💙
JD Mette
November 27, 2025 at 16:14I’ve been a pharmacist for 22 years. I report every time I see something unusual. But I’ve also seen patients who think reporting means they’ll get sued or that the drug company will come after them. That’s not true. The FDA doesn’t share personal info with manufacturers. The system is anonymous unless you choose to give your name. And honestly? The fact that you’re even considering this means you care more than most.
Don’t overthink it. Just describe what happened. Plain words. No jargon needed.
You’re not being a nuisance. You’re being a guardian.
Olanrewaju Jeph
November 28, 2025 at 14:25It is imperative to underscore the critical importance of accurate and timely adverse event reporting. The submission of incomplete or ambiguous data compromises the integrity of pharmacovigilance systems. For instance, failing to specify the exact dosage, frequency, or temporal relationship between drug administration and symptom onset may render a report scientifically inconclusive.
Therefore, I urge all reporters-regardless of professional background-to provide precise, chronological, and unambiguous details. Even if you are uncertain, document what you know. The FDA’s coders are skilled, but they cannot infer what is not stated.
Moreover, one must distinguish between adverse events and coincidental occurrences. While correlation does not imply causation, it does warrant documentation. The aggregation of such data over time enables pattern recognition that saves lives.
This system is not perfect, but it is indispensable. And it relies on the diligence of ordinary citizens. Your report is not merely a form. It is a contribution to the collective knowledge of human health.
Dalton Adams
November 30, 2025 at 05:55Look, I’ve read every single FDA guidance document on MedWatch since 2018. I’ve cross-referenced the 2023 safety bulletins with the WHO’s VigiBase and even analyzed the 2021 FDA label change dataset using Python. You people are missing the bigger picture.
MedWatch isn’t just a reporting tool-it’s a *signal extraction mechanism* in a high-noise environment. And most reports? They’re garbage. Half of them don’t even include the drug’s generic name. Some people write ‘I felt weird after taking the blue pill.’ That’s not a report. That’s a cry for help with zero data.
And don’t get me started on the supplements. People report ‘joint pain’ after taking ‘Turmeric Plus’-but they don’t list the other 11 ingredients. Do you know how many of those are laced with steroids? The FDA can’t trace it if you don’t give them the full label.
Also, why are you using the 3500B form if you’re a nurse? That’s like using a spoon to dig a trench. Use the right tool.
And for the love of science-stop saying ‘I think it was the drug.’ You either know the temporal sequence or you don’t. Be precise. Or don’t report at all. Half-measures are worse than silence.
Also, the mobile app pilot? It’s only in 15 hospitals. Don’t act like it’s public yet. You’re not special because you heard about it on Reddit. 🙄